Bringing Flow to the Battery World
What is a flow battery?
A redox flow battery (RFB) consists of three main spatially separate components: a cell stack, a positive electrolyte (shortened: posolyte) reservoir and a negative electrolyte (shortened: negolyte) reservoir.
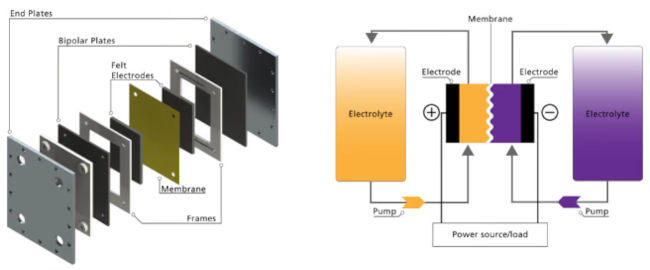
Flow battery cell (left) and redox flow battery system (right)
A cell stack is made up of several flow battery cells electrically connected in series, typically 50 cells. Electrolytes are the liquid media that contain energy storage particles known as reduction - oxidation (redox) active materials. An electrolyte is composed of redox active materials dissolved in a solvent known as the supporting electrolyte. The posolyte is analogous to the positive electrode (or pole) in a conventional battery cell while the negolyte is analogous to the negative electrode. A flow battery cell contains a membrane that prevents the mixing of the posolyte and the negolyte but allows charge carriers to flow across to complete the circuit. The cell stack and the electrolyte reservoir are connected through pipes, valves and pumps which shuttle the electrolyte between the stack and the reservoirs during the charge and discharge cycles. The pipes connecting the cells within the stacks are arranged in parallel to enable multiple flow paths without pressure loss.
How does it work?
The posolyte and the negolyte contain redox active particles with different tendencies to gain electrons (technical term: reduction) or lose electrons (technical term: oxidation). This tendency is referred to as the redox potential. The difference in redox potentials results in a potential difference across the battery terminals that causes current to flow in the external circuit. The chemical identity of a redox active particle changes during a reaction as the particle undergoes oxidation or reduction. This gives rise to a reduced and an oxidized state of a redox active species in each reservoir otherwise known as a redox couple.
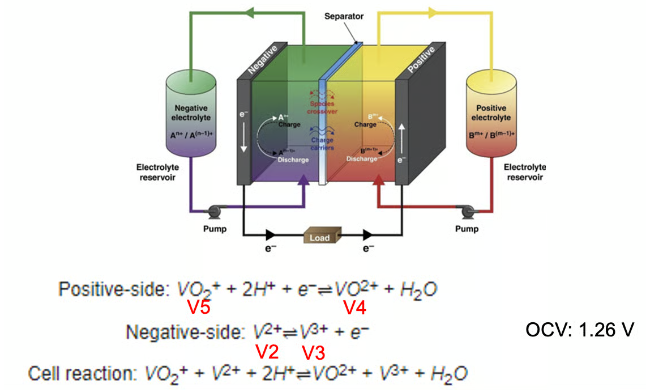
Vanadium redox flow battery charge and discharge reactions
Key differentiators
The genius of the RFB invention is the separation of power capacity and energy capacity. See this for a refresher on the difference between power and energy.
| Parameter | Conventional battery (lithium-ion, etc.) | Flow battery |
| Energy storage location | Inside a battery cell | In external reservoirs |
| Energy storage medium | Solid (electrodes) | Liquid (electrolytes) |
| Energy and power | Coupled | Independent |
Structural differences between a conventional battery and a flow battery
Contrary to a traditional cell, energy in an RFB is stored outside the cell. The number of cells within a stack determines the power capacity while the volume of the electrolyte reservoir determines the energy capacity of an RFB. Consequently, the two parameters can be altered independently (technical term: easily scalable energy-to-power ratio) - a feat not possible in conventional batteries such as lithium-ion batteries. The separation of energy and power yields benefits such as lower cost of incremental storage capacity (technical term: marginal cost of storage) and lower fire risks.
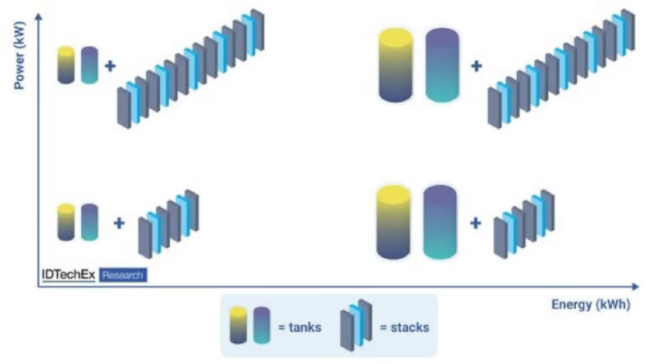
Decoupling of power and energy
How is a redox flow battery different from a conventional battery (lithium-ion, lead acid etc)?
- Energy is stored outside of the cell.
- Energy is stored in a liquid medium.
- Energy and power can be scaled independently.
Classification
RFBs are categorized based on the identity of the redox active particles (the technical term: RFB chemistry), the type of reactions at the electrodes (technical terms: pure vs hybrid RFB), whether the posolyte and the negolyte are similar or different (technical terms: symmetric vs asymmetric RFB), the nature of redox active particles (technical terms: organic vs inorganic RFBs) and the nature of the supporting electrolyte (technical terms: aqueous vs nonaqueous RFB).
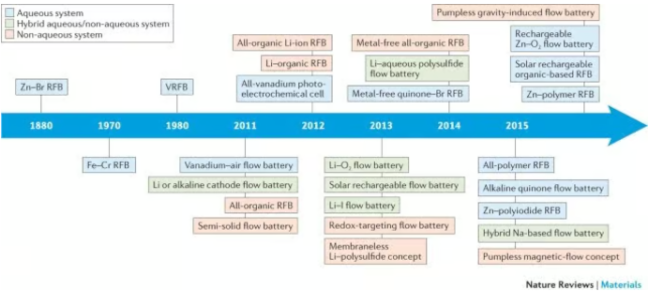
Flow battery classification and development timeline
Historical context
The earliest known RFB chemistry was a zinc-bromine RFB patented in the US circa 1879 by John Doyle. In the 1950s, an Estonian chemist by the name Walter Kangro demonstrated titanium - iron and iron - chromium RFBs. NASA would further develop and test the iron - chromium RFB in 1970 but the chemistry was not commercialized. In 1984, Maria Skyllas-Kazacos invented the breakthrough flow battery chemistry - the all vanadium RFB. This is a symmetric RFB that leverages the same electrolyte in both reservoirs by employing the existence of vanadium ions in 4 oxidation states. The 4 vanadium ions form two redox couples. The all vanadium RFB was the first RFB chemistry to achieve commercialization. The benefit of a symmetric RFB is it lowers the negative impacts of the mixing of the posolyte and the negolyte which happens inevitably since the membranes are not perfect at keeping the electrolyte particles apart.
Conclusion
In summary, a redox flow battery is a battery type in which energy is stored outside the battery cell. This has several advantages including easily scalable energy-to-power ratio, lower marginal cost (USD/kWh) and lower fire risk.
3/20/2024 2:00:00 PM
